ECG History | ECG Evolution | The EKG Glove | Glove Benefits | Instructions | The Future | Videos
The EKG Glove
The EKG Glove is a new, break-through, medical device that replaces the cumbersome cables, electrodes and plugs typically used to perform an electrocardiogram (ECG or EKG). The EKG Glove is disposable, portable and can easily connect to most industry standard ECG recording devices (including many of the new wireless systems) typically used in private practice, hospitals, emergency services and home health care.
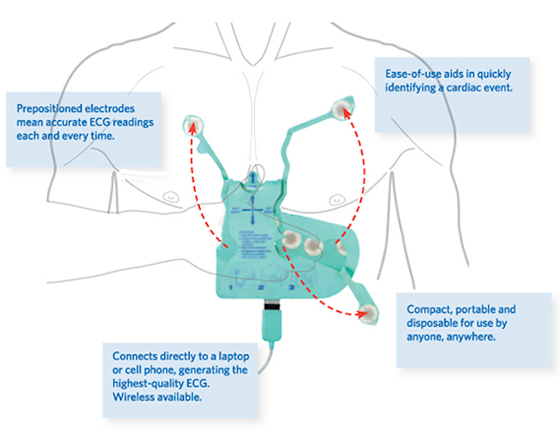
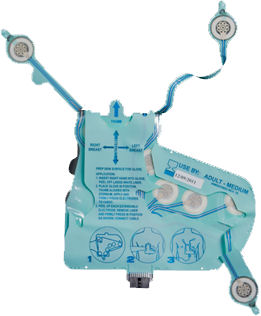
The EKG Glove has been designed to obtain an electrocardiogram (EGG) using 10 pre-positioned electrodes affixed to the underside of a glove-like sheath (or shell). The pre-printed conductive circuitry is contained within the sheath on a flexible substrate and never comes in contact with the patient.
To obtain the tracing, a care giver need only connect The EKG Glove to an ECG machine or wireless transmission device (using a single, compatible, multi-conductor cable*) then insert their right hand into The EKG Glove, properly position The EKG Glove over the patient's chest and then apply. Extending and attaching the three extendable electrodes will provide a full 12-Lead ECG recording.
The EKG Glove comes in 2 Adult sizes:
-
Medium: For use on patients with a chest girth between 97 - 104 cm.
-
Large: For use on patients with a chest girth greater than 104 cm.
*A compatible accessory cable is required to attach The EKG Glove to your equipment. Click here to see a list of ECG devices that we currently have compatible cables and/or connectors for.
FDA Cleared (USA) with CE Marking (EU)
The FDA cleared (510k) the pre-positioning of the 10 electrodes within The EKG Glove and has determined that the placement of these electrodes will produce measurements that can be used to generate a full 12 lead ECG that is substantially equivalent to an ECG created using conventional wired leads and traditional electrode placement as required in clinical electrocardiography. In the United States, The EKG Glove is currently classified as a Class II medical device. Prescription required.
 The
EKG Glove recently received CE Marking approval for resale within the European
Union. The EKG Glove is considered a class I medical device and can be sold
without a prescription to patients that live in countries that are a part of the
European Union.
The
EKG Glove recently received CE Marking approval for resale within the European
Union. The EKG Glove is considered a class I medical device and can be sold
without a prescription to patients that live in countries that are a part of the
European Union.
Research, Development and testing of The EKG Glove...
In addition to the extensive amount of internal research IneedMD, Inc. has done to prove the reliability, time, cost, and life saving benefits of The EKG Glove, there are three additional third party studies that we have commissioned:
-
An FDA study conducted at Ben Gurion University in Tel Aviv, Israel. We got the FDA 510K approval based on this study.
-
An NYU Medical Center study (50 patients) comparing ECGs generated using The EKG Glove to those generated using traditional wired leads and an industry standard ECG machine. The study results found that ECGs generated using The EKG Glove were comparable to those generated using wired leads and an industry standard ECG machine.
-
We just completed a 100 patient comparative study between The EKG Glove and 10 wired leads connected to a GE manufactured ECG machine (considered the “gold standard” ECG machine in most U.S. hospitals) at the department of Interventional Cardiology, at Mt. Sinai Hospital in New York City. World renowned cardiologist Dr. Samin Sharma is the chief of that department and oversaw the execution and completion of this study. Although early indicators suggest that the results are positive, the final report is still pending as of January 31st, 2012.
All three studies cited above, support the clinical validity of The EKG Glove as a credible, user friendly and sanitary “front end” alternative to the combination of cables, electrodes and plugs used with clinical quality ECG recording devices.
FDA:
- Product Cassification DRX
- 510(k) Premarket Notification
- FDA 510(k) Current Record for K033559
- 510(k) Summary
- CFR - Code of Federal Regulations Title 21
Patent Protected Technology
The EKG Glove is protected by seven (7) U.S. patents and several international patents.
- Patent # 6,224,548 – Patent # 6,248,064 - Patent # 6,540,673 - Patent # 6,595,918 - Patent # 7,112,175
- Patent # 7,435,222 - Patent # 7,753,845
ECG History | ECG Evolution | The EKG Glove | Glove Benefits | Instructions | The Future | Videos